Abstract
Background:
Vitamin C is an essential water-soluble vitamin required for many redox reactions in our body and its deficiency causes scurvy, a well characterized disease with multiple hematological manifestations. Studies dating back to 1950's demonstrated that patients with myeloid neoplasms tend to have lower plasma levels of vitamin C than healthy controls. Recent studies have shown that as much as 80% of patients with hematological malignancies in a cohort from Denmark had low vitamin C levels. Myeloid neoplasms tend to harbor mutations in epigenetic regulators which play a role in DNA methylation. One such mutation commonly seen in myeloid neoplasms and clonal hematopoiesis of indeterminate potential (CHIP) is TET2 for which vitamin C serves as a cofactor. There is a scarcity of clinical data on patients with low vitamin C level in myeloid neoplasms. Our study investigated the rates of vitamin C deficiency and the disease clinical and genomic characteristics associated with it at our center.
Methods:
We retrospectively collected data from a prospectively maintained list of patients treated for myeloid neoplasms at a large tertiary cancer center on whom vitamin C levels where serially collected during the study period. We obtained multiple baseline characteristics at the time of diagnosis including cytogenetic and molecular mutational data. Baseline characteristics were defined using descriptive statistics. Categorical variables were compared using a Fisher's exact test and continuous variables were analyzed using Mann Whitney U test for statistical significance. Institutional review board approval was obtained for the study. Statistical analysis was done using R Studio version 1.4.1717.
Results:
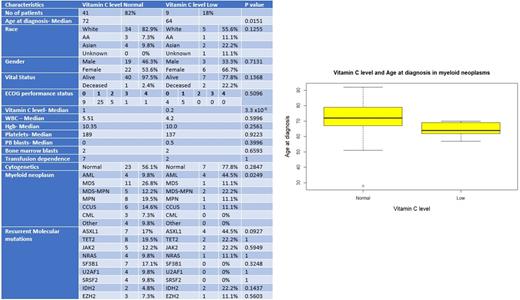
A total of 50 patients with myeloid neoplasms were identified with vitamin C levels available at least once during the study period. Nine (18%) patients had a low vitamin C level (LOW) defined as less than 0.4 mg/dl as per the Mayo lab testing with a reference range between 0.4 to 2.0 mg/dl. Baseline characteristics of patients with low vitamin C level and patients with normal vitamin C level (NORMAL) are shown in Table 1. The median vitamin C level in the LOW group was 0.2 mg/dl and NORMAL group was 1 mg/dl (p <0.001). The median age at diagnosis for patients in the LOW cohort was 64 years compared to 72 years for patients with normal vitamin C level (p = 0.015). Twenty-two (53.6%) of patients were female in the NORMAL cohort while six patients (66.7%) were females in the LOW cohort (p=NS). In the vitamin C LOW group only 55% of the patients were white compared to 83% in the NORMAL group (p = 0.093). The majority of patients in the Vit C LOW group had acute myeloid leukemia (AML) 44.5%, compared to 9.8% in the group with normal vitamin C levels (p = 0.03). Median white blood cell count, platelet counts, peripheral blast count and bone marrow blast count were not statistically significant amongst the 2 groups. Majority of patients in both groups 56.1% (NORMAL) vs 77.8% (LOW) had normal cytogenetics at the time of diagnosis (p = 0.284). There was a higher tendency to harbor ASXL1 and IDH2 mutation in the cohort with LOW levels 44.5% (p = 0.09) and 22.2% (p value = 0.143) compared to 17% and 4.8% respectively in the NORMAL cohort.
Conclusions:
Our analysis of the baseline characteristics of patients with myeloid neoplasms with vitamin C levels reveals interesting findings including a lower age at diagnosis for patients with low vitamin C levels and higher proportion of patients with acute myeloid leukemia compared to the cohort with normal levels. We also noted a higher tendency for occurrence of certain molecular mutations including ASXL1 and IDH2 among the patients with low vitamin C level. With recent papers implicating the role of ASXL1 in leukaemogenesis these findings suggest the hypothesis that vitamin C deficiency could accelerate clonal evolution with a higher tendency to transform into acute leukemia at a lower age. Further multi-institutional studies are needed to understand the relevance of low vitamin C level in myeloid neoplasms and the role of therapeutic vitamin C supplementation to retard leukaemogenesis.
Patel: Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; PVI: Honoraria. Awan: Cardinal Health: Consultancy; Abbvie: Consultancy; Merck: Consultancy; Beigene: Consultancy; Johnson and Johnson: Consultancy; Astrazeneca: Consultancy; BMS: Consultancy; Janssen: Consultancy; Genentech: Consultancy; Dava Oncology: Consultancy; Verastem: Consultancy; ADCT therapeutics: Consultancy; Incyte: Consultancy; MEI Pharma: Consultancy; Karyopharm: Consultancy; Kite pharma: Consultancy; Celgene: Consultancy; Gilead sciences: Consultancy; Pharmacyclics: Consultancy. Anderson: Celgene, BMS, Janssen, GSK, Karyopharm, Oncopeptides, Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Madanat: Blue Print Pharmaceutical: Honoraria; Stem line pharmaceutical: Honoraria; Onc Live: Honoraria; Geron Pharmaceutical: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal